This paper was published in 2007 and is included here for historical reasons. Many of the procedures and methods have been replaced as a result of more recent work. The review of the physiology of oviposition is still accurate.
——————————————
Some options to induce oviposition in turtles
Mark L. Feldman
Box 285, Kerikeri, Northland, New Zealand, 0230
E-mail: nz.feldman@yahoo.com
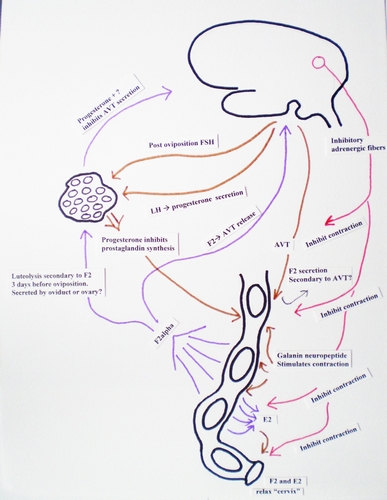
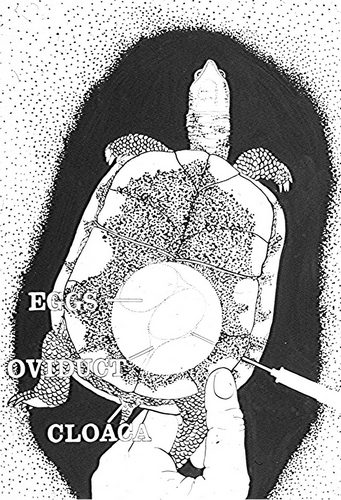
Abstract- From 1978 to 2007, oviposition was induced in 13 North American turtle species. Of the 245 inductions, 195 involved the use of oxytocin alone, 22 used arginine vasotocin (AVT) alone, 13 used a combination of oxytocin and ketamine, eight combined propranolol and oxytocin, and seven propranolol and AVT. The relative viability of Chrysemys picta picta eggs obtained from natural nests and via oxytocin induction was also evaluated. The oxytocin induced eggs were as viable as natural nest eggs. Suggested dosage ranges for oxytocin used alone vary from 0.7-4.0 units per 100 grams depending on species. In species where over 28 animals were injected with the suggested dosage, all eggs were laid after the first injection between 74% and 82% of the time, depending on species. With a second injection all eggs were laid between 83% and 94% of the time, depending on species. It would be desirable to find a combination of easy to use drugs that yielded a higher success rate with the initial injection, especially for species with a history of not responding to oxytocin. Although only small numbers (13 animals) were involved, there was a suggestion that the combination of ketamine and oxytocin may prove more effective than oxytocin alone. There was one significant side effect observed with oxytocin induction. Three days to two weeks after induction, some captive turtles without oviductal eggs displayed nesting behavior by digging completed nest holes and then remaining at the site for two-three hours before abandoning the hole. This side-effect might increase the risk of predation or auto trauma to wild animals after treatment with oxytocin. It might be avoided by using a more physiological drug combination to induce oviposition than oxytocin alone. Natural oviposition is complex and, at least, involves the interaction of peripheral beta-adrenergic neurons, AVT and prostaglandin F2a (PGF). Other, more physiologic approaches to induce oviposition, might be to use a beta-adrenergic blocker with oxytocin or PGF, PGF+oxytocin, PGF+ ketamine, or oxytocin + ketamine.
Key words.- arginine vasotocin; beta-adrenergic blocker; clutch; dosages; egg viability; hatching rate: hormones; induction; intraperitoneal injection; ketamine; oviposition; oxytocin; progesterone; propranolol; prostaglandin F2a ; turtle; viability
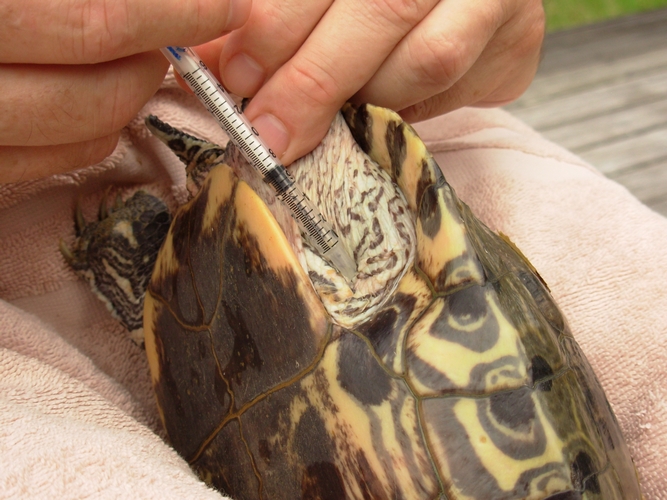
The use of oxytocin to induce egg laying in turtles has gained popularity since reports by Yntema, 1964, Ewert and Legler, 1978 and Ewert, 1979. Today oxytocin is widely used by veterinarians to treat “egg bound” turtles, by breeders, and by researchers to obtain eggs for experimental purposes and head-start programs. Despite this wide spread usage, the dosage recommendations still vary from 5 – 40 units/kg (DeNardo, 1996, Ewert et al., 1978). Since Ewert and Legler’s article in 1978 there have been no additional published studies evaluating the efficacy and safety of oxytocin induction in turtles.
This study was begun in 1978 as an attempt to narrow down the range of these dosage recommendations and to see if sensitivity to oxytocin was species specific. Because of the limited and unpredictable numbers and species of animals available each season, I used an empirical approach over a 28 year period to gradually clarify what the most effective dose would be for each species evaluated.
More recently Tucker et al. (in review CCB), working with large numbers of wild Trachemys scripta elegans and following an experimental protocol, determined that a dose of 10 units/kg produced the best results with the first injection. There has only been one study comparing the viability of naturally occurring nest eggs and eggs obtained by oxytocin injection (Wilgenbusch et al., 2000) and that showed comparable hatching rates for Chelydra serpentina.
Oxytocin is a pituitary hormone of mammals. There are numerous preparations available for human and veterinary use and it is available worldwide. Oxytocin is stable for years at room temperature and is very inexpensive. The reptilian and avian equivalents of oxytocin are arginine vasotocin (AVT) and mesotocin (Archer et al., 1972). AVT is ten times more potent than oxytocin and sixteen times more potent than mesotocin in isolated oviducts (LaPointe, 1977). However, AVT is very difficult to work with because it breaks down rapidly at room temperature, it must be kept frozen until use, and it retains its potency only if diluted just before injection (increasing the potential for dosage errors). AVT is also expensive and more difficult to obtain than oxytocin because of legal requirements. Dosage recommendations vary widely in the literature: Lloyd, 1990, suggested 0.01-1.0 ng/g and Mahmoud et al., 1987, used 4.6 ng/kg with Chelydra serpentina.